Describe the Differences Between Electrons Protons and Neutrons
Every solid liquid gas and plasma is composed of neutral or ionized atoms. Additionally protons help bind the atoms nucleus together.
Cbse 9 Chemistry Ncert Solutions
Firstly describe the fundamental differences between protons neutrons and electrons in terms of mass and charge.
. The mass of the neutron is considered as one unit and it equals the mass of a proton. Electrons are electrochemically negatively charged particles that move random around the nucleus. Protons and neutrons are hadronsparticles that are made up of quarks.
A particle with a negative charge -1 neutron. An atom has the same number of electrons and protons while the number of neutrons is variable. On the other hand Neutrons are symbolised as.
Together the protons and Neutrons make up the mass of the atom. A particle with a positive charge 1 electron. They have a relatively small mass compared to Protons and Neutrons.
Number of protons number of electrons atomic number Number of neutrons mass number atomic. Difference between Proton and Neutron in tabular form Conclusion The main difference between proton and neutron is that proton is a positively charged particle whereas neutron is an uncharged particle. Protons have a plus one charge neutrons or zero and electrons have a minus one charge.
If electrons involved in bonding spend most of the time closer to one Adam rather than the other the bond is. -The electrons have a negative charge and subatomic particles are those even lighter atoms. The three valence quarks in the proton include two up quarks and a down quark while the neutron valence quarks include two downs and an up.
According to charge symmetry an up quark in the proton acts like a down quark in the neutron and the most direct high-energy test of this property. Protons are positively charged electrons are negatively charged and neutrons are neutral. Basic Electrical Basic Electronics Difference between Physics.
-The neutrons are those particles which have no. Secondly explain how the range in air and penetrating powers of alpha beta gamma and x-rays are related to their nature and properties. The number of neutrons is equal to the difference between the mass number of the atom M and the atomic number Z.
The protons and neutrons are concentrated in the atomic nucleus while the electrons are distributed in the crust or the periphery of the atom. The charge of an electron is -1e which approximates to -1602 10-19. A particle with a neutral charge.
Protons and neutrons in the nucleus. The mass of an electron is about 12000 times the mass of a hydrogen atom. But the protons along with neutrons form the nucleus of the atom and are present at the center of the atomic nuclei.
On the other hand a neutron is charged. Secondly explain how the range in air and penetrating powers of alpha beta gamma and x-rays are related to their nature and properties. Key Differences Between Electron and Proton An electron is a negatively charged component of an atom whereas the proton is a positively charged body.
You can use these numbers to calculate the number of protons neutrons and electrons in an atom. 1st 1 is charged. Electrons and a compact nucleus of protons and neutrons.
What is the difference between protons and electrons. However Protons are symbolised as p. Determine if the elements in the following compounds are metals or non-metals.
Terms in this set 5 proton. The mass of an electron is approximately 91 10-31. Protons have a positive.
Different isotopes of an element have the same number of protons but vary in the number of neutrons present in their respective nuclei. Neutrons are electrically neutral particles and carry no charge. Protons and neutrons 3.
The mass number of the atom M is equal to the sum of the number of protons and neutrons in the nucleus. Within the atom the positively charged protons attract the negatively charged electrons and help to keep them in orbit. These quizzes will quiz you on what the difference and definition of protons electrons and neutrons are.
The nucleus consists of positively charged protons and neutral subatomic particles known as neutrons. Atoms are extremely small typically around 100 picometers across. An atom is the smallest unit of ordinary matter that forms a chemical element.
The electrons are present outside the nucleus in the orbiting shells. Protons neutrons and electrons can all be characterized and compared based on three primary characteristics. Electrons are NOT part of the nucleus of an atom they only encircle it typically called the electron cloud.
However a Proton is charged positively. Protons are electrochemically positive in charge and the Neutrons are electrochemically neutral in charge. Main Differences Between Electron Proton and Neutron The electron is charged negatively.
The mass of the proton is taken as one unit and equals the mass of a neutron. The quark sea contains additional quark flavors. The number of electrons in a neutral atom is equal to the number of protons.
2 days agoIonic Bond between a Metal and Non-Metal M NM Covalent Bond between a Non-Metal and Non-Metal NM NM PART 1. Electrons are symbolised as e. Firstly describe the fundamental differences between protons neutrons and electrons in terms of mass and charge.
These particles determine the characteristics and properties of the chemical elements. -The protons are positively charged and its weight is 1836 times that of the electrons. Electrons are outside the nucleus and mass protons and neutrons have about a massive 1 am.
They are present in the outer shells within an atom and orbit the positively charged nucleus in well-defined orbits. Protons and neutrons remain at the nucleus the ultra-dense center of the atom while electrons occupy the outer shell that is mostly empty space.
Difference Between Proton Neutron And Electrons

Difference Between Electrons Protons And Neutrons Science Structure Of The Atom 13920774 Meritnation Com

What Is In An Atom What Is The Difference Between Protons Neutrons And Electrons The Three Main Subatomic Particles Are Distinguished By Mass Charge Ppt Download
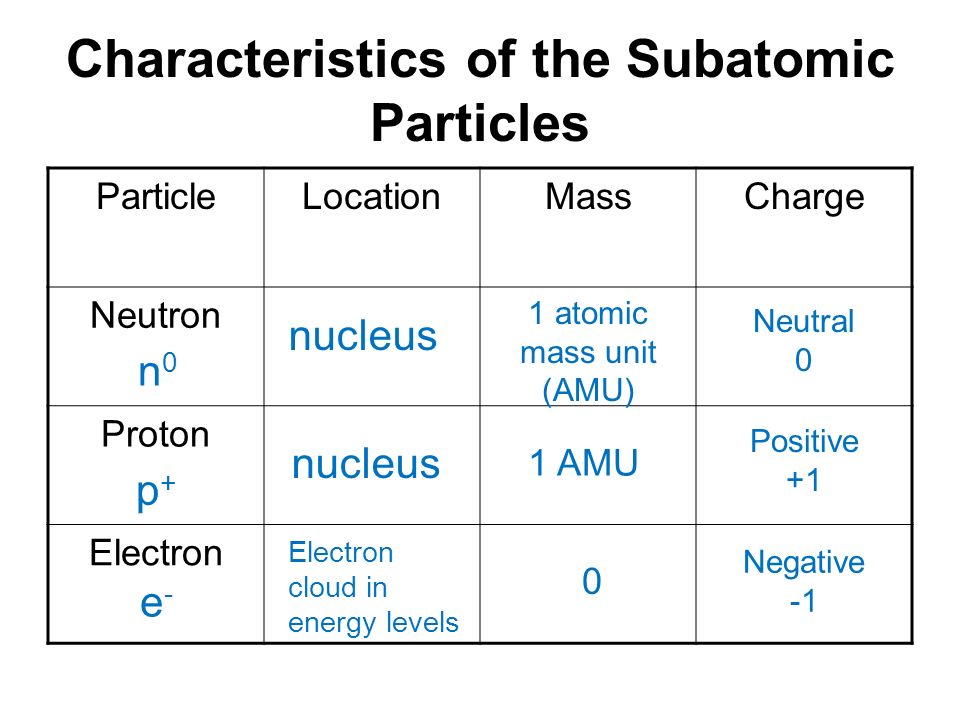
What Are The Characteristics Of Electron Proton And Neutron A Plus Topper
No comments for "Describe the Differences Between Electrons Protons and Neutrons"
Post a Comment